What Are Proteolysis-Targeting Chimeras (PROTACs)? Mechanisms of Action and Advantages
Table of Contents
In drug development, researchers frequently face targets that prove tough to adjust with standard methods. Numerous disease-related proteins do not possess enzymatic functions, display smooth interaction areas, or quickly change amid selection forces. Proteolysis-Targeting Chimeras (PROTACs) have surfaced as a workable option instead of just an idea.
On a practical level, research in PROTACs heavily depends on reliable proteins, antibodies, biochemical assays, and pathway analysis tools. Established in 2004, Beijing Solarbio Science & Technology Co., Ltd. has built an extensive platform of life science reagents that supports work in molecular biology, cell biology, and immunology. Through its integrated research solutions for drug discovery and protein studies, Solarbio provides practical support to researchers investigating protein degradation pathways and PROTAC-related mechanisms.
What Are Proteolysis-Targeting Chimeras (PROTACs)?
PROTACs represent a shift in the design of therapeutic compounds. Rather than merely inhibiting protein function, they facilitate the complete elimination of target proteins from the cellular environment. This fundamental distinction explains why PROTACs are increasingly regarded as a distinct class of therapeutics, rather than simply a variant of conventional inhibitors.
Bifunctional Chimeric Molecule Design
A PROTAC is a bifunctional chimera composed of a target-binding ligand, an E3 ligase-binding ligand, and a chemical linker. This structure enables a single small molecule to tether a disease-causing protein to the cellular protein degradation machinery. Consequently, PROTACs have become valuable tools in small-molecule drug discovery, offering a means to modulate proteins at the post-translational level.
Difference Between PROTACs and Traditional Inhibitors
Standard inhibitors rely on sustained binding to inhibit protein activity. In contrast, PROTACs initiate a degradation cycle and subsequently dissociate, enabling them to catalyze multiple rounds of degradation. This catalytic nature allows PROTACs to maintain efficacy even when protein levels rise, a scenario commonly encountered during prolonged treatment. Consequently, they provide a more durable and reliable strategy for long-term protein modulation.
Research Tools Supporting PROTAC Studies
The rigorous evaluation of PROTACs necessitates access to high-quality recombinant proteins, well-characterized antibodies, and precise protein detection assays. Solarbio provides an extensive portfolio of research-grade reagents for protein and immunology studies, including biochemical kits and antibodies that are widely referenced in reputable scientific literature. These resources enable the systematic assessment of degradation efficacy across diverse experimental systems. Solarbio reagents have been employed in over 150,000 high-impact publications, with more than 18,000 SCI-indexed papers citing our products in 2023 alone. This established record ensures that PROTAC researchers utilize validated tools endorsed by the global scientific
How Do PROTACs Degrade Specific Proteins?
The real power of PROTACs comes from their skill in using an already present cellular route, rather than adding made-up ways to break down proteins. This method fits well with the natural cycle of protein replacement in cells.
Recruitment of the Ubiquitin-Proteasome System
PROTACs function through the ubiquitin-proteasome system (UPS), the primary pathway for selective protein degradation in eukaryotic cells. By facilitating the attachment of ubiquitin chains to a target protein, PROTACs mark it for recognition and degradation by the proteasome. This mechanism underlies their classification as chemical inducers of degradation rather than conventional inhibitors. In essence, PROTACs harness the cell’s intrinsic degradation machinery to efficiently eliminate target proteins.
Formation of the Ternary Complex
For degradation to happen well, a firm ternary complex must form, which includes the target protein, the PROTAC, and an E3 ligase. The joint effects inside this group often count more than just how strongly things bind, and this has altered the ways researchers examine mechanisms of action of Proteolysis-Targeting Chimeras. Therefore, studies now focus on how these parts work together in real cell conditions.
Experimental Validation and Detection Methods
Scientists usually verify protein breakdown with methods like Western blot analysis, ELISA, and assays that measure enzymes in linked steps. Solarbio supplies pathway-focused reagents and biochemical assay kits that aid in precisely tracking protein reduction in different experimental frameworks. These resources ensure that results remain consistent and trustworthy during testing phases.
What Is the Role of E3 Ligases in PROTACs Mechanisms?
E3 ligases set the precision in the ubiquitin-proteasome pathway. Picking the correct one greatly affects if a PROTAC succeeds in cells or falls short.
Commonly Used E3 Ligases in PROTAC Design
Just a few E3 ligases see regular use because of available binding agents and their fit with biology. Selecting the best ligase usually follows the needs of the disease, not just ease of chemistry. This careful choice helps tailor PROTACs to specific health issues effectively.
E3 Ligase Expression and Tissue Specificity
The levels of E3 ligases differ between body tissues, and that is why a PROTAC might do great in one kind of cell but not in another. As such, checking expression patterns and analyzing pathways becomes a vital first move. Researchers use these checks to predict how well a PROTAC will perform in various biological settings.
Advantages of PROTACs in Targeted Therapy
Targeting Protein-Protein Interactions
PROTACs can break apart signaling groups by taking out one key part, and this creates fresh paths for targeted therapy. Such actions disrupt networks that drive disease without needing to hit every piece.
Challenges and Solutions in PROTAC Development
Molecular Size and Cell Permeability
PROTACs tend to be bigger than usual small molecules, and this can impact how well they enter cells and move through the body. Thus, smart design of linkers stays crucial for success.
Off-Target Degradation Risks
The chance of breaking down the wrong proteins persists as a worry. Tools like proteomic screening and checks on pathways assist in lowering these dangers.
Solarbio solutions in PROTAC research
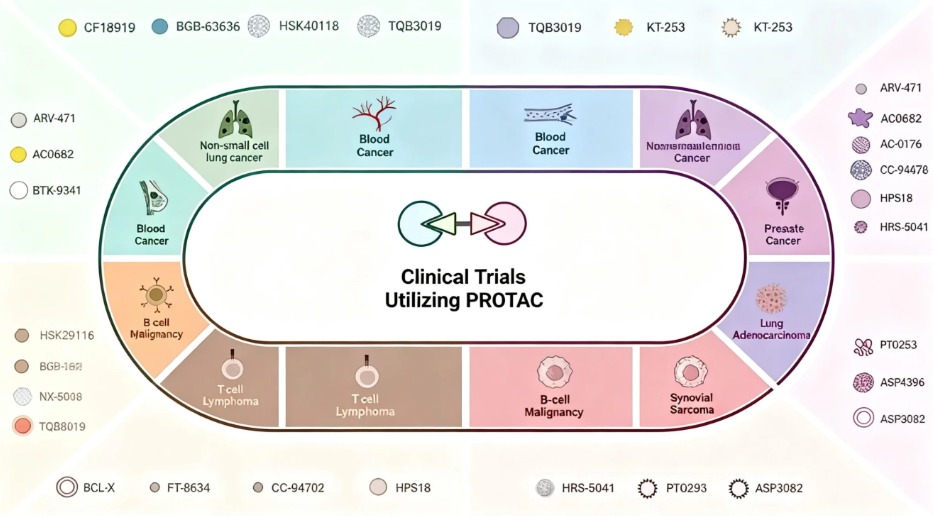
To facilitate researchers in conducting such studies, Solarbio small molecule compounds have launched the PROTAC/protein degradation-targeting chimeric molecule series. Currently, there are already more than a thousand ROTAC/protein degradation-targeting chimeric molecule products available, and the PROTAC/protein degradation-targeting chimeric molecule series is still being gradually updated and improved.
FAQ
Q1: What Are Proteolysis-Targeting Chimeras (PROTACs)?
A: PROTACs are bifunctional molecules that induce selective protein degradation by recruiting the ubiquitin-proteasome system.
Q2: How Do PROTACs Work to Target Disease-Causing Proteins?
A: They form a ternary complex with an E3 ligase, leading to ubiquitination and proteasomal degradation of the target protein.
Q3: What Is the Role of E3 Ligases in PROTACs Mechanisms?
A: E3 ligases determine degradation specificity and strongly influence tissue response and selectivity.