AMPK Signaling Pathway
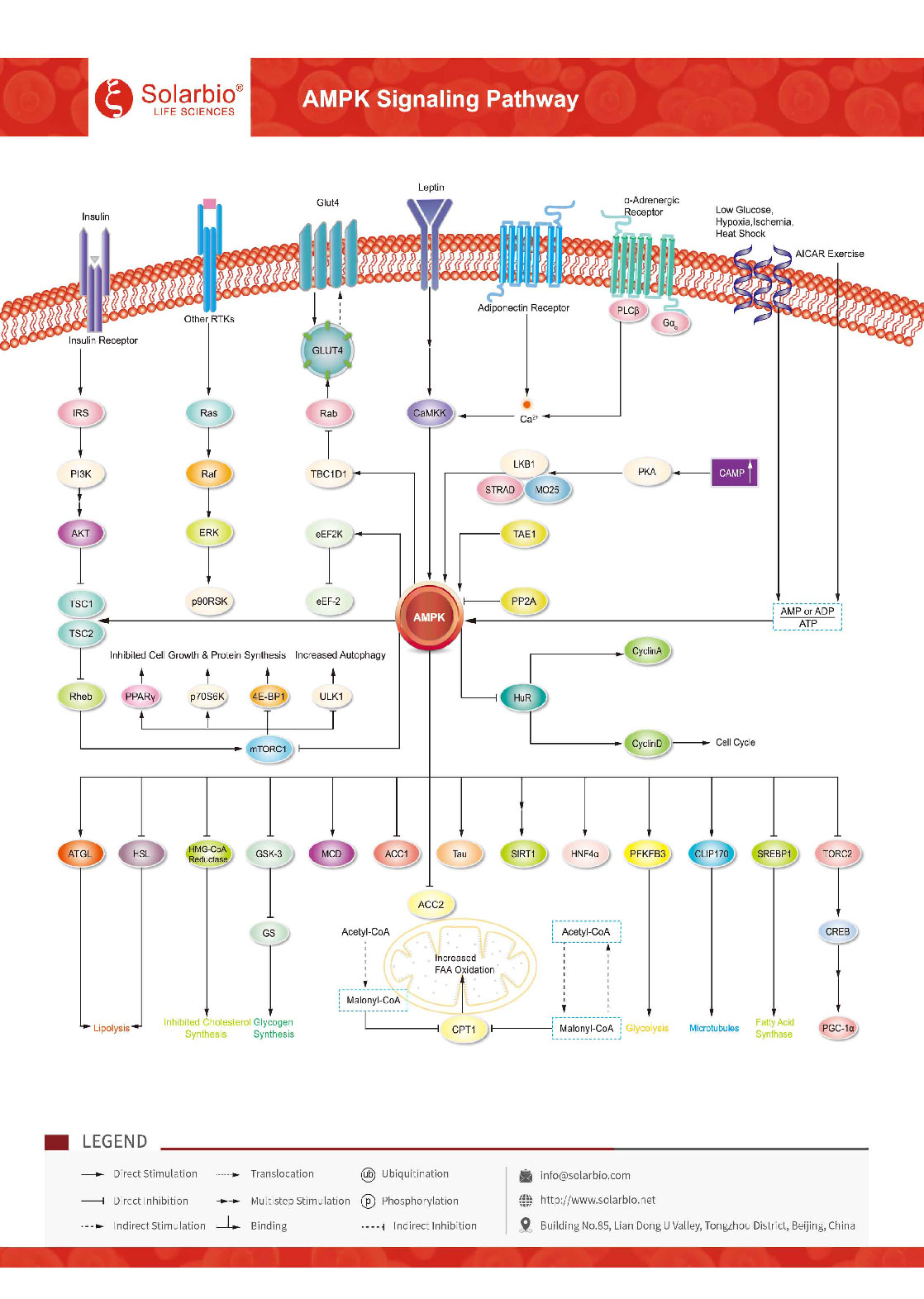
AMPK (AMP-activated protein kinase), an AMP-dependent protein kinase, is a serine-threonine kinase that is highly conserved during evolution. It is a key molecule in the regulation of biological energy metabolism and is required for the body to maintain glucose balance. AMPK is activated by an increase in the AMP/ATP ratio in the body and by any metabolic stress that disrupts energy balance by interfering with ATP synthesis, such as low glucose levels, tissue hypoxia, ischemia, and heat shock. AMPK functions as a metabolic master switch regulating several intracellular systems, including cellular uptake of glucose, β-oxidation of fatty acids and glucose transporter 4 (GLUT4) and mitochondrial biogenesis. Some upstream kinases, such as Liver Kinase B1 (LKB1), Calcium/Calmodulin kinase kinase-beta (CaMKK beta), and TGF-beta-activated kinase-1 (TAK-1), activate AMPK by phosphorylating threonine residues on its catalytic α subunit. Once activated, AMPK leads to inhibition of energy-consuming biosynthetic pathways, such as protein, fatty acid, and glycogen synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation and glycolysis. AMPK is expressed in various metabolism-related organs and can be activated by various stimuli, including cellular stress, exercise, and many hormones and substances that can affect cell metabolism. Genetic and pharmacological studies have shown that AMPK is necessary for the body to maintain glucose balance and is central to the study of diabetes and other metabolism-related diseases.