Blood- Brain Barrier Signaling Pathway
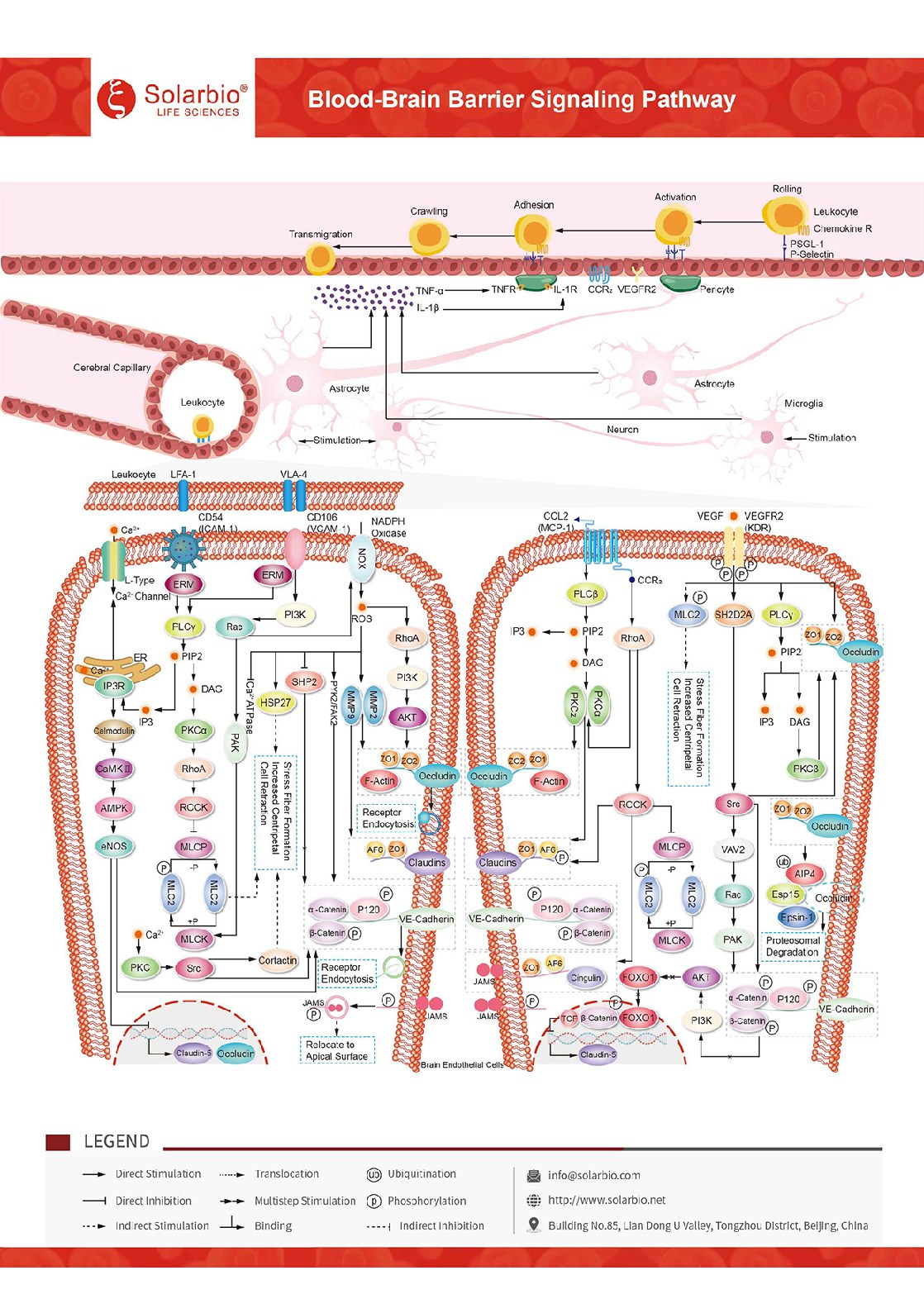
The Blood-Brain Barrier is a robust barrier within the cerebral microvasculature. It includes a physical barrier composed of specialized endothelial cells (ECs) consisting of apical tight junctions (TJs), mid-adheres junctions (AJs), and mid-basolateral Ca2+-dependent adheres, as well as a metabolic barrier supported by enzymes and efflux pump proteins. Together, they form a highly selective diffusion barrier between the peripheral circulation and the central nervous system (CNS). Chemokine CCL2/MCP-1 is a key chemokine regulating monocyte/macrophage migration and infiltration. In the central nervous system under the state of neuroinflammation, CCL2/MCP-1 increases and binds to the G protein-coupled receptor CCR2 on brain ECs. By inducing the phosphorylation of TJ proteins, CCL2/ McP-1 initiates an intracellular signaling cascade to disintegrate and/or redistribute TJ proteins from the cell edge, causing dynamic reorganization of junctional complexes and EC contraction. JAM proteins will reorient to the apical surface of endothelial cells and participate in adhesive interactions with leukocyte integrins. Intercellular adhesion molecule-1 (ICAM-1/CD54) An Ig-like adhesion receptor that binds to leukocyte integrin αLβ2 (LFA-1) induces ICAM-1/CD54 aggregation and activation of intracellular signaling pathways, including Src family kinases and Rho/ROCK pathways. In addition, ICAM-1/CD54 signaling promoted the endocytosis of Occludin and VE-Cadherin. Vascular cell adhesion molecule-1 (VCAM-1/CD106) is an Ig-like adhesion receptor and a ligand for leukocyte integrin α4β1 (VLA-4). Aggregation of VCAM-1/CD106 and activation of intracellular signaling pathways that induce TJ protein phosphorylation lead to disassembly and/or redistribution of TJ proteins from the cell edge, which in turn mediate the migratory process of leukocytes from the endothelium into tissues through the blood circulation. In addition, VCAM-1/CD106 signaling promotes the production of matrix metalloproteinases (MMPs), which degrade connexin. VEGF is a highly specific pro-vascular endothelial cell growth factor. In a state of neuroinflammation, VEGF is elevated in the CNS, binds to receptors on brain ECs, and initiates intracellular signaling cascades that occur, causing dynamic reorganization of junctional complexes and EC contraction. VEGF promotes the endocytosis of Occludin and VE-Cadherin. Alternatively, VEGF induces the degradation of occludin through the ubiquitin-proteasome pathway. VEGF could also down-regulate the expression of Occludin and Claudin-5. Under normal conditions, the specialized structure of the BBB prevents paracellular transport of most hydrophilic compounds through brain endothelial cells and limits the migration of blood-derived cells to the central nervous system. In the process of neuroinflammation, the junctional complexes such as TJs and AJs can be reshaped to form endothelial gaps, through which peripheral immune cells will enter the central nervous system (CNS). CCL2/MCP-1, ICAM-1/CD54, VCAM-1/CD106, and VEGF signals can all induce the reorganization of F-actin microfilaments into stress fibers, thereby increasing the centritropic tension in cells and leading to cell contraction. Prolonged tissue injury can trigger inflammation and cause the blood-brain barrier to lose its restrictive function. Reactive microglia, astrocytes, pericytes and endothelial cells release a large number of molecules (such as CXCL8/IL-8, CCL2/MCP-1, TNF-α, IL-1beta/IL-1F2, etc.), leading to increased vascular permeability. It promotes the invasion of peripheral immune cells into the central nervous system. Blood-brain barrier dysfunction and subsequent increase in infiltrating immune cells have been reported to be associated with a variety of neurological diseases, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis.