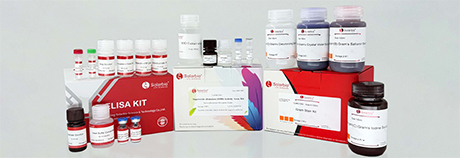
C-type Lectin Receptor Signaling Pathway
C-type lectin receptors (CLRs), a superfamily of proteins containing C-type lectin like domain (CTLD), are mainly expressed in myeloid cells. The main type Ⅱ CLRs present on dendritic cells (DCs) include: Dcs-specific intercellular adhesion molecule 3 binding non-integrin (DC-SIGN), macrophage inducible C-type lectin (Mincle), DCS-associated C-type lectin-2 (Dectin-2), blood DCs antigen-2 (BDCA-2), DCs immune receptor (DCIR) and DCs immune activation receptor (DC) AR) and so on. Dectin-1, Dectin-2, and Mincle are three well-characterized pathogen-recognized CLRs that induce canonical NF-κB signaling, MAPK signaling, and NFAT-dependent transcription. After the extracellular sugar recognition domain (CRD) of C-type lectins binds ligands, the intracellular CRD recruits downstream proteins through phosphorylation and activates the downstream immune signaling pathway through SYK. SYK can activate IκB kinase (IKK) and multiple mitogen-activated protein kinases (MAPK), including p38, JNK and ERK1/2, by stimulating a multiprotein complex consisting of CARD9, Bcl-10 and MALT1 through activation of PKCδ. IKK subsequently phosphorylates IκB and promotes nuclear translocation of NF-κB, whereas p38, JNK, and ERK1/2 phosphorylate and activate AP-1. DC-SIGN activates the serine/threonine kinase Raf-1. Raf-1 translocation to the nucleus leads to phosphorylation of the NF-κB subunit p65 at serine. Finally, p65 is acetylated at multiple lysine sites to regulate the transcription level of IL-10. CLRs stimulate intracellular signaling cascades that induce the production of inflammatory cytokines and chemokines, thereby triggering both innate and adaptive immunity to pathogens.