MAPK-p38 Signaling Pathway
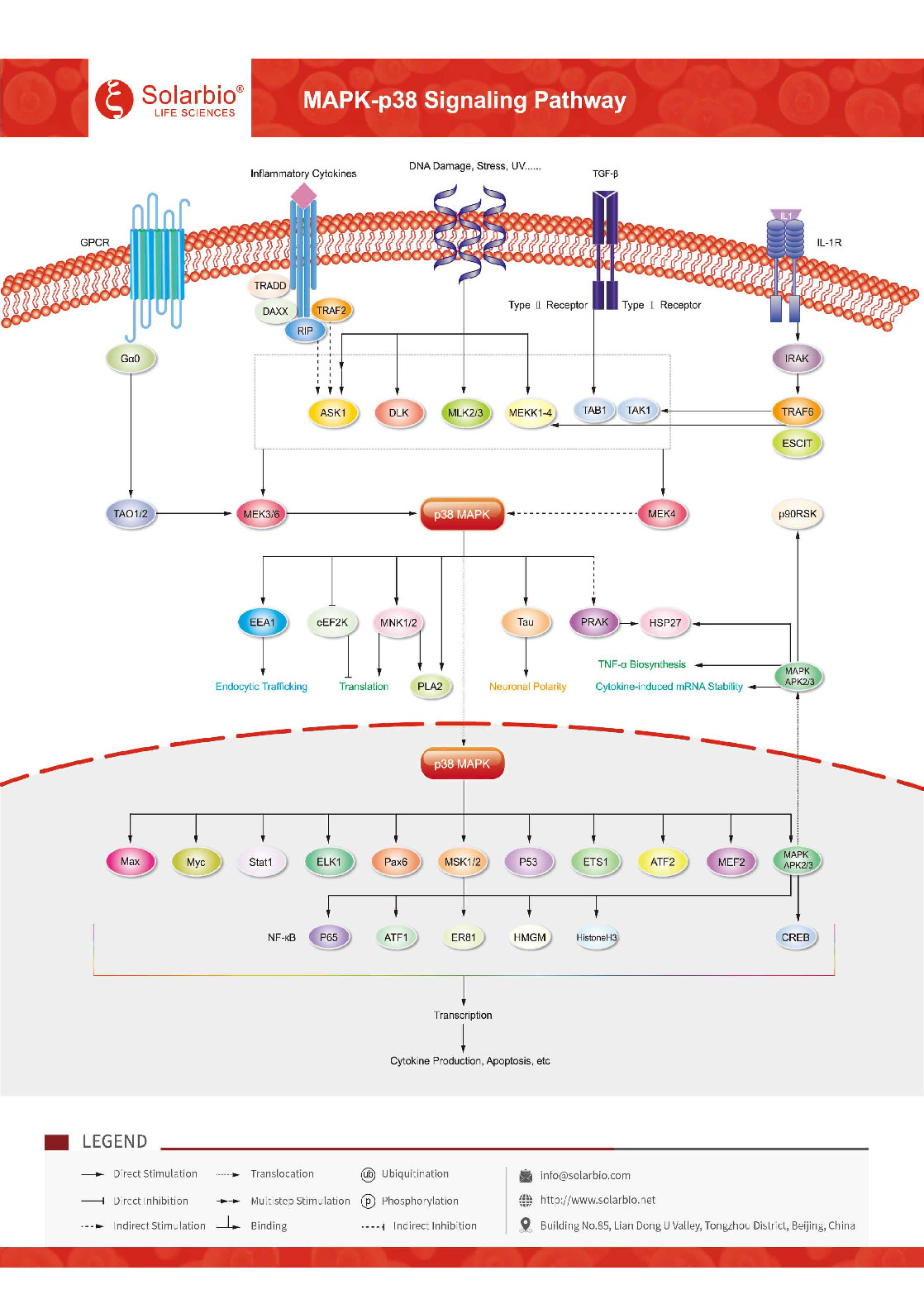
Mitogen-activated proteinkinases (MAPKs) are a class of serine/threonine proteinkinases which are involved in a variety of cellular functions, including cell proliferation, differentiation and migration. The MAPKs signaling pathway is highly conserved in cell evolution. Several parallel MAPKs signaling pathways have been found in lower prokaryotic cells and higher mammalian cells. Different extracellular stimuli can use different MAPKs signaling pathways to mediate different cell biological responses. p38 MAPK (α, β, γ, and δ) is a member of the MAPK family consisting of highly conserved proline-directed serine-threonine protein kinases that are activated in response to a variety of growth factors, cytokines, and chemotactic substances (e.g. vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF)). PDGF, TNF, interleukin, lipopolysaccharide (LPS) and formyl-methionyl-leucyl-phenylalanine (fMLP). It is well known that p38 mediates inflammation and apoptosis, thus becoming a target for the development of anti-inflammatory drugs. In the p38 MAPK signaling pathway, MAPKKK phosphorylates and activates MKK3/6, which is a p38 MAPK kinase. MKK3/6 can also be directly activated by ASK1 in response to the stimulation of apoptotic factors. p38 MAPK is involved in the regulation of HSP27, MAPKAPK-2 (MK2), MAPKAPK-3 (MK3) and several other transcription factors, including ATF-2, Stat1, Max/Myc complex, MEF-2, and Elk-1, while CREB is indiretly regulated through activation of MSK1