Growth Factors
Classification and Characteristics
-
Platelet-Derived Growth Factor (PDGF)
PDGF is composed of two subunits, referred to as the A chain (18 kDa) and the B chain (12–14 kDa), which share highly homologous peptide sequences, including 8 conserved cysteine residues at identical positions. PDGF exists in 3 isoforms: the homodimers PDGF-AA and PDGF-BB, and the heterodimer PDGF-AB. These isoforms are distributed across various tissues and cell lines, suggesting distinct biological functions for each.
2 Types of PDGF receptors are present on the cell surface:PDGFR-α and PDGFR-β. PDGFR-α can bind both A and B subunits of PDGF, whereas PDGFR-β binds only the B subunit. PDGF-AA binds to αα receptor dimers, PDGF-AB can form αα and αβ dimers, and PDGF-BB can activate αα, αβ, and ββ receptor dimers. These receptor dimers represent the active signaling forms of PDGFR [1].
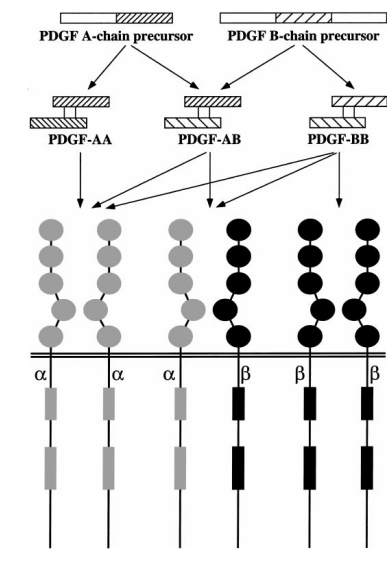
Figure 1: Processing and Functions of Platelet-Derived Growth Factor (PDGF) Isoforms [2]
-
Vascular Endothelial Growth Factor (VEGF)
Vascular Endothelial Growth Factor (VEGF), also known as Vascular Permeability Factor (VPF), is a highly specific mitogen for vascular endothelial cells. The VEGF family includes several members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and Placenta Growth Factor (PlGF). Among them, VEGF-A exists in four main isoforms—VEGF121, VEGF165, VEGF189, and VEGF206—produced through alternative mRNA splicing.
VEGF exerts its biological effects by binding to three main types of tyrosine kinase receptors: VEGFR-1 (Flt-1), VEGFR-2 (Flk-1/KDR), and VEGFR-3 (Flt-4). Each VEGF isoform exhibits different binding affinities toward these receptors, which contributes to the diversity of VEGF-mediated signaling pathways [3].
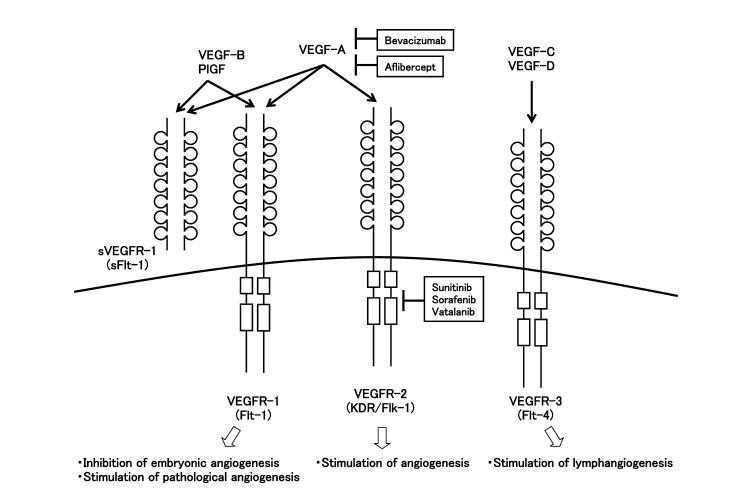
Figure 2: VEGF Family Members and Their Receptors
VEGF-A is generally referred to simply as VEGF and serves as a key regulator of developmental vasculogenesis, angiogenesis, and the differentiation of endothelial progenitor cells. VEGF-B plays a role in tumors where neovascularization is absent. VEGF-C and VEGF-D are involved in the formation of both new blood vessels and lymphatic vessels within tumor tissues. VEGF-E is also considered a potential angiogenic factor, while PlGF (Placenta Growth Factor) promotes neovascularization and increases vascular permeability.
-
Epidermal Growth Factor (EGF)
Epidermal Growth Factor (EGF) is a small polypeptide widely present in humans and other animals, consisting of a peptide chain with 50–60 amino acids. This chain contains 6 cysteine residues that form stable disulfide bonds, resulting in 3 looped domains critical for its biological activity. Even at extremely low concentrations, EGF can powerfully stimulate cell growth, inhibit the expression of aging-related genes, and delay epidermal cell senescence.
EGF exerts its effects by binding to the extracellular region (comprising Domains I–IV) of its receptor, EGFR (Epidermal Growth Factor Receptor), leading to receptor tyrosine kinase dimerization [4]. This activation triggers a cascade of biochemical events, including elevated intracellular calcium levels, enhanced glycolysis and protein synthesis, and increased expression of certain genes—among them the EGFR gene itself. Ultimately, these events promote DNA synthesis and cell proliferation.
-
Fibroblast Growth Factors (FGFs)
Fibroblast Growth Factors (FGFs) are a family of polypeptide growth factors present in various tissues throughout the body. They exist primarily in 2 closely related forms: basic FGF (bFGF) and acidic FGF (aFGF). The FGF family consists of 22 ligands that can interact with four distinct FGF receptors (FGFRs). The FGF/FGFR signaling pathway governs fundamental cellular processes such as cell survival, proliferation, migration, differentiation, embryonic development, organogenesis, tissue repair/regeneration, and metabolism [5].
The name “fibroblast growth factor” reflects their potent mitogenic effect on fibroblasts. In addition, FGFs also stimulate the proliferation of smooth muscle cells, chondrocytes, keratinocytes, and pericytes. Due to their strong affinity for heparin, FGFs are also known as heparin-binding growth factors. They are synthesized by vascular endothelial cells and are stored within the basement membrane and extracellular matrix.
-
Insulin-like Growth Factors (IGFs)
The insulin-like growth factor (IGF) family comprises 2 low-molecular-weight polypeptides (IGF-1 and IGF-2), 2 types of specific receptors (IGF-1R and IGF-2R, also referred to as Type I and Type II receptors), and 6 IGF-binding proteins (IGFBPs). IGF-1R shares a structural similarity with the insulin receptor (IR), forming a heterotetrameric glycoprotein (α₂β₂) composed of 2 α-subunits and 2 β-subunits. The α-subunit contains the ligand-binding domain, while the β-subunit possesses intrinsic tyrosine kinase activity (but no tyrosinase activity).
The binding affinities of insulin and IGFs to their respective receptors vary:
- For the insulin receptor (IR): Insulin > IGF-1 > IGF-2
- For the IGF-1 receptor (IGF-1R): IGF-1 > IGF-2 > Insulin
- For the IGF-2 receptor (IGF-2R): IGF-2 > IGF-1, with no cross-reactivity observed for insulin.
IGF-1 is a single-chain basic protein composed of 70 amino acids, with a molecular weight of approximately 7,649 Da and notable heat stability. IGF-2, on the other hand, is a single-chain slightly acidic protein with 67 amino acids and a molecular weight of about 7,471 Da, and is stable in 0.1% SDS. The 2 share over 70% sequence homology and approximately 50% structural and functional similarity to human proinsulin.
-
Transforming Growth Factors (TGFs)
Transforming Growth Factors (TGFs) refer to 2 classes of polypeptide growth factors: TGF-α and TGF-β. TGF-α is produced by macrophages, brain cells, and epidermal cells, and plays a role in inducing epithelial development.
In humans, TGF-β exists in three isoforms: TGF-β1, TGF-β2, and TGF-β3. These isoforms bind to a shared receptor complex composed of TGF-β receptor type I (TGF-βR1) and type II (TGF-βR2), inducing similar intracellular signaling cascades in vitro [6].
TGF-β (Transforming Growth Factor Beta) is a multifunctional protein that regulates a wide range of cellular processes, including cell growth, differentiation, apoptosis, and immune modulation. TGF-β receptors are serine/threonine kinase receptors, and downstream signaling is primarily mediated through the SMAD pathway and/or the DAXX pathway.
-
Connective Tissue Growth Factor (CTGF)
Connective Tissue Growth Factor (CTGF), also known as FISP12 or CCN2, belongs to the CCN family of proteins, which includes 3 members: Cyr61, CTGF, and Nov. These family members exhibit a high degree of amino acid sequence homology and share a conserved protein structure comprising four key domains:
- an N-terminal insulin-like growth factor-binding domain (IGFBP),
- a von Willebrand factor type C repeat (VWC),
- a thrombospondin type 1 repeat (TSP1),
- and a cysteine-rich C-terminal domain (CT).
CTGF was first identified in 1991 as Fibroblast-Inducible Secreted Protein-12 (FISP12), isolated from serum-activated NIH3T3 cells. The name “Connective Tissue Growth Factor” was proposed later that same year.
CTGF was initially recognized for its ability to promote fibroblast proliferation, migration, adhesion, and extracellular matrix (ECM) production. Each of its structural domains can bind specific partner proteins, enabling CTGF to perform a wide range of biological functions [7] (see Figure 3).
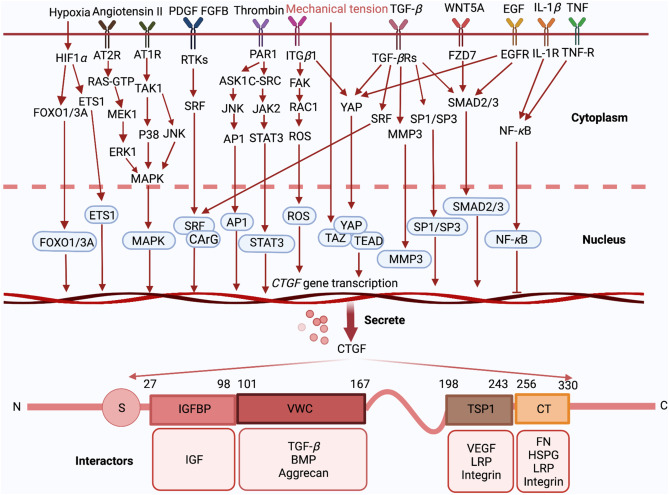
Figure 3
Upon external stimulation, CTGF-related signaling pathways are activated, leading to CTGF transcription and secretion. After secretion, the four distinct domains of CTGF—IGFBP, VWC, TSP1, and CT—can interact with various molecules to exert their physiological functions.
-
Keratinocyte Growth Factor (KGF)
Keratinocyte Growth Factor (KGF) is a basic protein growth factor secreted by mesenchymal cells in subcutaneous tissues. It specifically stimulates various physiological processes in epithelial cells, including metabolism, regeneration, differentiation, and migration. KGF is a naturally occurring, bioactive, soluble protein in the human body, encoded by a sequence of 194 amino acids. The mature form of KGF consists of 163 amino acid residues and contains an N-terminal glycosylation site.
KGF is primarily secreted by subcutaneous tissue and binds specifically to its receptor on the surface of epithelial cells. Through a complex signaling cascade, it activates the expression of genes involved in cell division and growth, thereby promoting the metabolic activity and regeneration of epithelial tissues.
-
Nerve Growth Factor (NGF)
The nerve growth factor (NGF) family includes several neurotrophic factors: NGF itself, brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), neurotrophin-4/5 (NT-4/5), and, to a lesser extent, neurotrophin-6 and neurotrophin-7. Among them, the first four are the most prominent and well-studied.
NGF is the most essential and representative member of this family. In tissues, NGF is primarily present in a precursor form, which is processed into its mature form mainly in the submandibular gland. NGF plays multiple roles, including supporting neuronal survival, protecting nerves, promoting nerve regeneration, modulating immune responses, facilitating wound healing, and inhibiting tumor growth.
In addition to the aforementioned growth factors, other important members include interleukin-related growth factors (such as IL-1 and IL-3), erythropoietin (EPO), and colony-stimulating factors (CSFs). These molecules also play critical roles in regulating cell proliferation, differentiation, and immune responses within various physiological and pathological contexts.
Mechanism of Action
-
Modes of Action
Endocrine: Growth factors are secreted and transported via the bloodstream to act on distant target cells. An example is platelet-derived growth factor (PDGF).
Paracrine: Growth factors secreted by a cell act on nearby cells of a different type, influencing their behavior.
Autocrine: Growth factors act on the same cell that synthesized and secreted them.
Among these, paracrine and autocrine signaling are the predominant modes of action for most growth factors.
-
Mechanistic Pathway
After being secreted by various cell types, growth factors (GFs) exert their biological effects by binding to specific receptors located on the surface or within target cells. Upon ligand binding, these receptors activate intracellular signaling cascades that mediate the corresponding biological responses.
A major class of growth factor receptors possesses intrinsic tyrosine kinase activity and is known as receptor tyrosine kinases (RTKs). RTKs are divided into approximately 20 families, including the epidermal growth factor receptor (EGFR) family, platelet-derived growth factor receptor (PDGFR) family, and nerve growth factor receptor (NGFR) family. The actions of most growth factors are mediated through RTKs (see Figure 4).
When a growth factor binds to its receptor on the cell surface, signal transduction is initiated by the dimerization of the RTKs and subsequent activation of their kinase domains. The two monomers within the RTK dimer undergo cross-phosphorylation on tyrosine residues, fully activating the receptor. These phosphorylated tyrosine residues on the C-terminal tails of the RTKs serve as docking sites for multiple downstream signaling molecules.
The formation of these RTK signal complexes leads to the activation of various downstream signaling pathways, including the Ras/Erk, PI3K/Akt, Src/Jak/Stat, and PLC-γ1 pathways. These pathways interact with one another to form a complex signaling network [8].
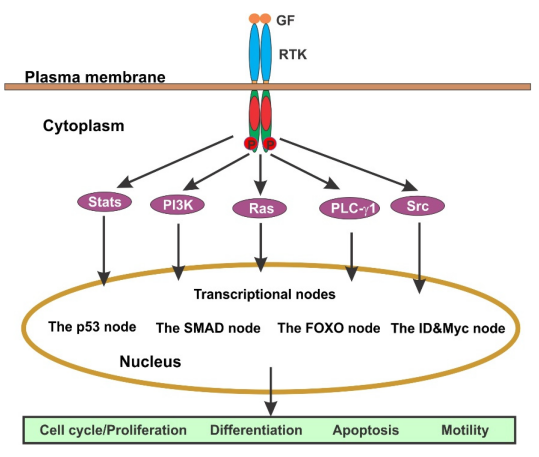
Figure 4
RTK–Ras Signaling Pathway:
Ligand binding → Activation of receptor tyrosine kinase (RTK) → Activated RTK recruits adaptor proteins → Guanine nucleotide exchange factor (GEF) promotes GDP release → Activation of Ras (a GTP-binding protein).
This activation triggers a cascade of downstream events:
Activated Ras initiates the activation of Raf, a serine/threonine protein kinase (also known as MAPKKK), which phosphorylates serine/threonine residues on target proteins → Activated Raf binds to and phosphorylates MAPKK (MAPK kinase), leading to its activation. MAPKK is a dual-specificity kinase capable of phosphorylating both threonine and tyrosine residues on MAPK, thereby activating MAPK → Activated MAPK translocates into the nucleus → Phosphorylation of other kinases or gene regulatory proteins (i.e., transcription factors), modulating gene expression (see Figure 5).
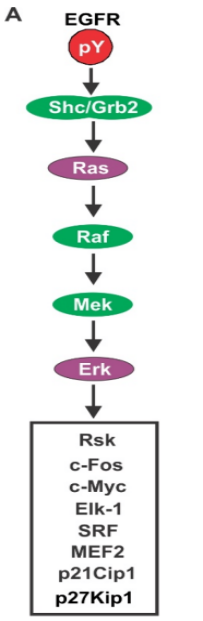
Figure 5
References
1 Kaji K. Function, molecular structure and gene expression regulation of Platelet-derived growth factor. Nihon Rinsho. 1992 Aug;50(8):1902-1909.
2 Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999 Oct;79(4):1283-1316.
3 Takahashi S. Vascular endothelial growth factor (VEGF), VEGF receptors and their inhibitors for antiangiogenic tumor therapy. Biol Pharm Bull. 2011;34(12):1785-1788.
4 Ogiso H, Ishitani R, Nureki O, et al. Crystal structure of the complex of human epidermal growth factor and receptor extracellular domains. Cell. 2002 Sep 20;110(6):775-787.
5 Mossahebi-Mohammadi M, Quan M, Zhang JS, et al. FGF Signaling Pathway: A Key Regulator of Stem Cell Pluripotency. Front Cell Dev Biol. 2020 Feb 18;8:79.
6 Sun T, Vander Heiden JA, Gao X, et al. Isoform-selective TGF-β3 inhibition for systemic sclerosis. Med. 2024 Feb 9;5(2):132-147.e7.
7 Fu M, Peng D, Lan T, et al. Multifunctional regulatory protein connective tissue growth factor (CTGF): A potential therapeutic target for diverse diseases. Acta Pharm Sin B. 2022 Apr;12(4):1740-1760.
8 Wang Z. Regulation of Cell Cycle Progression by Growth Factor-Induced Cell Signaling. Cells 2021, 10, 3327.
Related Products:
If you want to get more details, please go to our Store website: www.solarbio.com.
|
Cat No. |
Product Name |
Cat No. |
Product Name |
|
SEKH-0052 |
Human VEGF ELISA Kit |
SEKH-0050 |
Human EGF ELISA Kit |
|
SEKH-0316 |
Human TGF-β1 ELISA Kit |
SEKM-0035 |
Mouse TGF-β1 ELISA Kit |
|
SEKM-0039 |
Mouse VEGF ELISA Kit |
SEKR-0032 |
Rat VEGF ELISA Kit |
|
SEKR-0010 |
Rat EGF ELISA Kit |
SEKR-0012 |
Rat TGF-β1 ELISA Kit |